- Your cart is empty
- Continue shopping
Shop
Citalopram for anxiety
$200.00 – $300.00
Citalopram for anxiety is a drug whose action is directed at selective blockade of serotonin reuptake in synapses of neurons of the central nervous system. Under the influence of the drug Citalopram, there is a decrease in obsessive conditions, an improvement in mood, elimination of anxiety, overexertion, and fears, as well as dysphoria. The use of this medication practically does not provoke the development of a sedative effect.
Citalopram for anxiety
Citalopram for anxiety is a drug from the group of antidepressants, selective serotonin reuptake inhibitors. Buy Citalopram Online
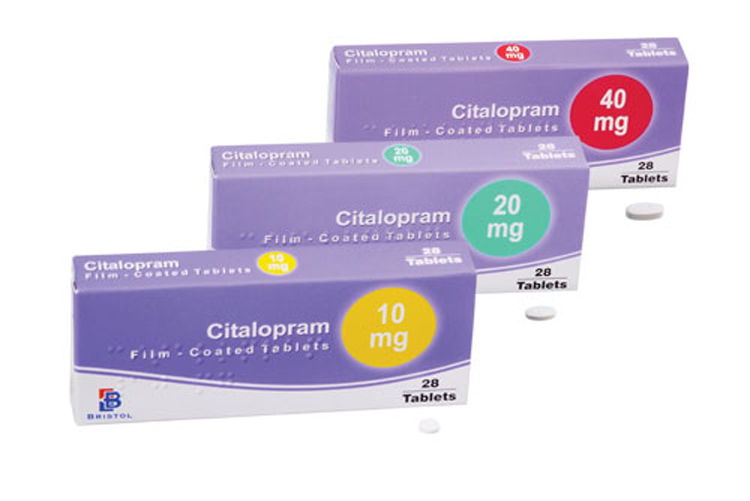
Form release, composition: Citalopram is presented in the pharmaceutical market in the form of tablets for internal administration at a dosage of 20 and 40 mg. The active ingredient is citalopram hydrobromide.
PHARMACHOLOGIC EFFECT
Citalopram for anxiety is a drug whose action is directed at selective blockade of serotonin reuptake in synapses of neurons of the central nervous system. Under the influence of the drug Citalopram, there is a decrease in obsessive conditions, an improvement in mood, elimination of anxiety, overexertion, and fears, as well as dysphoria. The use of this medication practically does not provoke the development of a sedative effect.
A strong clinical effect is achieved after 1 week – 10 days of use of tablets. Antidepressant effect can be achieved after 2 weeks – 1 month of regular use of the drug.
After the internal administration of the drug, the maximum concentration is achieved after 2-4 hours. Excretion of the active component is carried out by the kidneys and intestines.
In patients with liver dysfunction, the clearance of the active component of the drug is reduced to 37%.
CITALOPRAM FOR ANXIETY INDICATIONS
The drug Citalopram can be recommended for admission by a doctor when:
– Depressive states of various origins;
– development of bipolar and dysthymic disorders;
– Vascular dementia, accompanied by a depressed state;
– mixed anxiety-depressive disorders;
– psychosomatic pathologies accompanied by vegetative dysfunctions, including autonomic dysfunction of the respiratory, cardiovascular, and genitourinary systems, as well as the organs of the gastrointestinal tract;
– somatization disorders;
– anxiety-phobic disorders, accompanied by the development of phobias, panic, obsessive-compulsive, post-traumatic, anxious, and stressful conditions;
– Depressive conditions in women: during menopause and before menstruation, during pregnancy and after childbirth;
– eating disorders: the development of anorexia or bulimia;
– Depressive conditions, provoked by alcoholism;
– Depression in the elderly.
Before you start taking pills, you need a doctor’s consultation.
MODE OF APPLICATION
Citalopram for anxiety Tablets should be taken orally 1 time per day. The drug is not chewed and washed down with a small amount of water.
Therapy starts with a minimum effective dose, which can be further increased on the recommendation and under the supervision of the doctor. The exact dosage and duration of therapy can be determined only by a qualified specialist, given the indications for admission, the tolerability of the drug, and the characteristics of the patient’s body.
Patients with mild or moderate forms of chronic renal insufficiency do not show a dosage adjustment. Patients with severe forms of kidney failure should be especially careful when selecting the appropriate dosage.
The total duration of therapy, as a rule, is about six months.
Abrupt withdrawal of the drug Citalopram for anxiety can lead to nausea, dizziness, vomiting, headache, sleep disturbances, tremor, parasthesias, nervousness, and asthenic conditions.
To avoid the development of withdrawal syndrome, after the completion of treatment, the abolition of taking tablets should be carried out for several weeks, gradually reducing the dose of the drug. The period of cancellation is about 14 days, but in each case, this issue is considered individually. Some patients needed several months or more to stop using the medication.
USE DURING PREGNANCY
Citalopram can be used during the therapy of pregnant women in those cases when the potential benefit to the mother exceeds the possible risks to the fetus.
Experimental studies have not revealed a teratogenic effect of the drug on fetal development and reproductive function.
CONTRAINDICATIONS
Citalopram is contraindicated in receiving when:
– intolerance of the constituents of the drug;
– in case of simultaneous use with medicines from the group of MAO inhibitors;
– the drug should not be combined with medicines, the active ingredient of which is selegiline, moclobemide, linizolid, and also within 2 weeks after discontinuation of their use;
– Therapy of a drug from the MAO inhibitor group can begin no earlier than 1 week after the completion of the citalopram;
– simultaneous therapy with medications (including pimozide), contributing to the elongation of the QT interval on the electrocardiogram;
– Due to a lack of necessary information on the effectiveness and safety of the drug during the treatment of children, citalopram is not used in the treatment of patients under the age of 18 years.
Citalopram For Anxiety Side Effects
Against the background of the use of citalopram, unwanted side effects can be developed on the part of the organs of vision, urinary, cardiovascular, digestive, reproductive, respiratory, and nervous systems, as well as metabolism and skin. Increased risk of developing asthenic conditions, fainting, allergic manifestations, yawning, gnashing of teeth, dizziness, headache, atrial flutter, tachycardia, body weight fluctuations, rhinitis, sinusitis, and cough.
At the beginning of therapy, the patient may have complaints about insomnia, and a sense of anxiety. Such conditions can be eliminated by correcting the initial dose of the drug.
When an overdose increases the likelihood of drowsiness, episodic convulsions, coma, sinus tachycardia, increased sweating, nausea, hyperventilation, vomiting, and cyanosis.
There is no specific antidote, with the development of an overdose, symptomatic therapy is required and the use of funds for maintenance treatment is required.
Citalopram For Anxiety DRUG INTERACTIONS
When the drug Citalopram is with drugs from the MAO inhibitor group, there is a possibility of developing a serotonin syndrome (hypertensive crisis).
The active component of citalopram can lead to an increase in the effectiveness of drugs, the active component of which is sumatriptan, as well as other serotonergic drugs.
In the case of indirect use of anticoagulants with indirect drugs, as well as other means that contribute to the effects of blood coagulation (atypical antipsychotics, tricyclic antidepressants, nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, dipyridamole), there is a possibility of developing coagulation disorders. In this case, at the beginning and after the completion of the use of citalopram, blood coagulation.
When combined with medications based on warfarin, an increase in prothrombin time by 5% is possible.
Additional Recommendation for Citalopram for anxiety
If there is a risk of suicidal tendencies in patients who have a history of depression, you should carefully monitor these people at the beginning of therapy and prescribe the medication at the lowest effective dose to reduce the risk of overdose. This precaution should be followed when treating other types of psychological disorders due to the risk of simultaneous development of a depressive episode.
Children, adolescents, and people younger than 24 years with depression and other psychological pathologies constitute a risk group, because taking drugs from the group of antidepressants can contribute to the development of suicidal thoughts and behavior. Short-term studies have demonstrated that in patients over 24 years of age, the risk of suicide did not increase, and in persons over 65 years of age it declined.
During therapy with citalopram or other drugs from the group of antidepressants, these categories of patients should be carefully and systematically monitored to detect suicidal tendencies promptly.
Citalopram for anxiety should be used with extreme caution in those patients who have a history of renal insufficiency (with creatinine clearance below 30ml / min), hypomania, mania, diabetes mellitus, cirrhosis, bleeding tendency, pharmacologically uncontrolled epilepsy, depression accompanied by suicidal tendencies. Zeecontainer kopen
Also, caution requires simultaneous use of alcohol, with medications that help reduce the threshold of convulsive readiness, provoking hyponatremia. Hyponatremia can develop as a result of impaired production of antidiuretic hormone. The risk groups are older women.
The use of the drug may affect the level of glucose in patients with diabetes mellitus. Against the background of treatment with citalopram, the dose of insulin or hypoglycemic drugs.
In rare cases, the occurrence of an actusis was reported, which is characterized by the development of a permanent or recurring feeling of motor anxiety, manifested in the form of the patient’s inability to remain calm in one position or the duration to be without movement. Such a reaction can take place during the first few weeks of therapy.
Patients who have a history of bipolar disorders increase the risk of developing mania. The use of the drug in this case is recommended to be discontinued.
Citalopram for anxiety is used with extreme caution if the patient’s history has epileptic seizures or drug dependence. With the development of manic states, the taking of tablets is immediately stopped.
A sharp cessation of the use of a medicine may trigger a withdrawal syndrome. There is a possibility of developing dizziness, nausea, and headache. To avoid such reactions in the body, the abolition of the use of tablets should be carried out for several weeks.
Storage of tablets should be carried out in a dark place, protected from direct sunlight, and children with observe a temperature mode: of no more than 25 degrees.
Buspirone 10 mg, Saxenda weight loss, Contrave weight loss 8/90, Panbesy 30mg, Saxenda for weight loss
| Dose | 10mg, 20mg, 40mg |
|---|